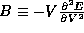 . The higher B is, the more difficult (greater energy
cost) it is to compress (decrease the volume of) the metal.
. The higher B is, the more difficult (greater energy
cost) it is to compress (decrease the volume of) the metal.
In this problem you will use your knowledge of quantum mechanics to
predict a measurable quantity which effects your daily life, the
resistance of metals to being compressed. The quantity which measures
this ``resistance'' is the bulk modulus 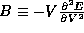 . The higher B is, the more difficult (greater energy
cost) it is to compress (decrease the volume of) the metal.
. The higher B is, the more difficult (greater energy
cost) it is to compress (decrease the volume of) the metal.
At the end of the problem, you will have the opportunity to compare your own quantum calculations with actual experimental results.
To perform this calculation, you will need to accept as given but one fact which we have not discussed yet in lecture. This one fact is the Pauli Exclusion Principle, which you learned about in elementary chemistry class. The principle states that in systems containing more than one electron, at most two (2) electrons (one spin-up and the other spin-down) may occupy the same quantum energy state at once.
You will make the simplifying assumption that the bulk modulus of a metal at room temperature is close enough to its modulus at zero temperature (T=0) so that for the purposes of this problem, you can assume that the electrons in a metal are all in their lowest energy configuration.
The notation used in this problem is as follows. You will consider a
cubic chunk of metal of side L and volume 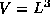 and containing N
electrons of mass m. We will call the volume density of electrons
and containing N
electrons of mass m. We will call the volume density of electrons
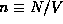 . The energies of individual electrons will be
denoted by the lower case Greek
. The energies of individual electrons will be
denoted by the lower case Greek  's; the energy of the entire
collection of electrons, the metal as a whole will be denoted with an
upper case Roman E. The symbols
's; the energy of the entire
collection of electrons, the metal as a whole will be denoted with an
upper case Roman E. The symbols 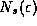 and
and
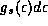 refer to the counting of states, just as they
did in our black body discussions, and the symbols
refer to the counting of states, just as they
did in our black body discussions, and the symbols 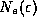 and
and
 refer to the counting of electrons. From the
Pauli principle then
refer to the counting of electrons. From the
Pauli principle then 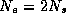 ,
, 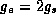 . As the electrons seek
the lowest possible energy configuration, they fill all available
energy states up to some maximum energy level which we will call
``
. As the electrons seek
the lowest possible energy configuration, they fill all available
energy states up to some maximum energy level which we will call
``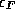 '' for historical reasons.
'' for historical reasons.  is known as the the
Fermi energy. (For those taking 8.044: the Fermi energy is just
the chemical potential
is known as the the
Fermi energy. (For those taking 8.044: the Fermi energy is just
the chemical potential 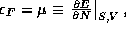 the energy cost of adding a single
particle to the system.)
the energy cost of adding a single
particle to the system.)