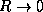 and
and 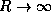 limits of your curves.
limits of your curves.
Skectch the energy of the electron in the molecule as a function of
R assuming that the electron is always in the lowest energy bound
state. Give physical explainations for the 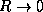 and
and 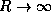 limits of your curves.
limits of your curves.
Skectch the total energy of the molecule, including the
internuclear repulsion, 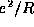 . Show that the two atoms prefer to
form a bond over being separated at infinite distance by considering
the separation
. Show that the two atoms prefer to
form a bond over being separated at infinite distance by considering
the separation 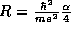 , where
, where 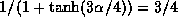 .
.