- (a)
- What is the wavelength of the electrons? Is it smaller or larger
than the width of the slit?
- (b)
- Sketch the flux of electrons as a function of the position on the
screen
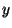 . (The point opposite the center of the slit is at
. (The point opposite the center of the slit is at 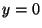 .) On the
same sketch, show the flux you would expect if electrons did not behave
as waves.
.) On the
same sketch, show the flux you would expect if electrons did not behave
as waves.
- (c)
- How far from
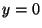 can one place an electron detector and still
detect a non-zero flux?
can one place an electron detector and still
detect a non-zero flux?
HINT: Recall problem set # 9, problem 5 (``Alone in the
Dark''.) Just like in that problem, you can assume that flux is zero outside
the central intensity maximum!
- (d)
- Electrons hitting the screen away from
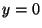 have a non-zero
momentum
in the
have a non-zero
momentum
in the 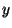 direction. Find
direction. Find 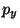 of an electron hitting the screen at a
point
of an electron hitting the screen at a
point 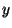 . With the same assumption as in part (c), what is the maximal
value of the magnitude of this momentum,
. With the same assumption as in part (c), what is the maximal
value of the magnitude of this momentum, 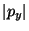 ? What is its minimal
value? The ``uncertainty'', or spread, in
? What is its minimal
value? The ``uncertainty'', or spread, in 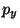 is defined as
is defined as
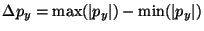 . Find
. Find
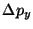 .
.
NOTE: Since the
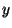 component of the electron momentum does not change on the way from the
slit to the screen,
component of the electron momentum does not change on the way from the
slit to the screen,
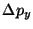 can also be thought of as the uncertainty
in the
electron momentum at the moment when it passes through the screen.
can also be thought of as the uncertainty
in the
electron momentum at the moment when it passes through the screen.
- (e)
- Observing an electron on the screen, we do not know exactly its
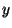 coordinate when it passed through the slit: it could be anywhere from
coordinate when it passed through the slit: it could be anywhere from
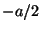 to
to 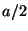 . Thus, the ``uncertainty'' in the position of the electron,
. Thus, the ``uncertainty'' in the position of the electron,
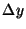 , is equal to the width of the slit
, is equal to the width of the slit 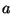 . Show that the uncertainties
in the coordinate of the electron and its position satisfy the relation
. Show that the uncertainties
in the coordinate of the electron and its position satisfy the relation
NOTE: Heisenberg uncertainty principle states that for
any physical system, 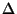 (coordinate)
(coordinate)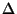 (momentum) is
at least
(momentum) is
at least 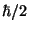 .
.
- (f)
- If the slit is made very narrow,
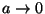 , what is the
expected uncertainty in momentum,
, what is the
expected uncertainty in momentum,
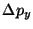 ? Based on this result, sketch
the expected flux of electrons as a function of the position on the screen for
such a narrow slit. Does the result agree with the expectation from our study
of wave interference pattern from one narrow slit?
? Based on this result, sketch
the expected flux of electrons as a function of the position on the screen for
such a narrow slit. Does the result agree with the expectation from our study
of wave interference pattern from one narrow slit?
![]() N
N![]() sec is directed
at a slit of width
sec is directed
at a slit of width ![]() nm. The electrons are then observed at a screen a
distance
nm. The electrons are then observed at a screen a
distance ![]() cm from the slit.
cm from the slit.
![]() component of the electron momentum does not change on the way from the
slit to the screen,
component of the electron momentum does not change on the way from the
slit to the screen,
![]() can also be thought of as the uncertainty
in the
electron momentum at the moment when it passes through the screen.
can also be thought of as the uncertainty
in the
electron momentum at the moment when it passes through the screen.
![]() (coordinate)
(coordinate)![]() (momentum) is
at least
(momentum) is
at least ![]() .
.