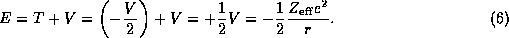To perform the classical analysis we will restrict ourselves here to considering circular orbits of radius r and will call the momentum of the electron about its orbit p. We leave the central charge an ``effective" charge to allow for ``shielding" of the nucleus in the case of many-electron atoms.
The first stage in the classical analysis is to replace the concerted motion of the nucleus and electron with the motion of their relative coordinate (the vector from the nucleus to the electron), which then moves as though it had an effective mass

Note that the effective mass is very close to the mass of the electron
(because 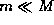 ), a basic reflection of the fact that the nucleus,
being so massive, hardly moves and the motion is almost entirely due to
the electron.
), a basic reflection of the fact that the nucleus,
being so massive, hardly moves and the motion is almost entirely due to
the electron. 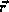 now becomes the distance between the electron
and
the nucleus and
now becomes the distance between the electron
and
the nucleus and 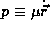 is the momentum of
the relative coordinate.
is the momentum of
the relative coordinate.
Next we balance the centripetal acceleration and the central force
(note the use of the effective mass 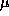 throughout),
throughout),

(Note that this is usually written 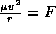 ) which gives
our first relation
) which gives
our first relation

which may be used to eliminate p in any expression in terms of r,

We may use this to find immediately the period of the motion in terms of the orbital distance
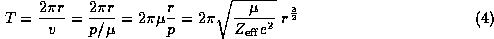
which we keep for future reference.
The last stage of the classical analysis is to consider the energy of the system. We have both kinetic and potential energies

Note that from (2) we have a special case of the the Virial theorem for orbital motion 2 T = -V and so we may eliminate p from (5) and find the energy in terms of the orbital distance alone,