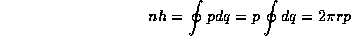
With the classical analysis complete, we now perform the quantization procedure. In this case we have found all quantities in terms of the classical orbital distance r and so all that remains is to find the allowed quantized values of r. The condition (1) gives, because the momentum is constant around an orbit,
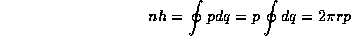
But because from (3)
 ,
we may eliminate p and solve for r,
,
we may eliminate p and solve for r,
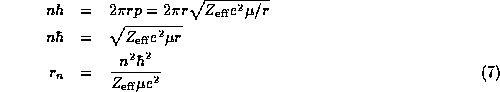
where we have given r a subscript n indicating its value in the
 quantum state. Now that we have the allowed radii,
we also have the allowed energies from (6),
quantum state. Now that we have the allowed radii,
we also have the allowed energies from (6),

(8) is often also written as
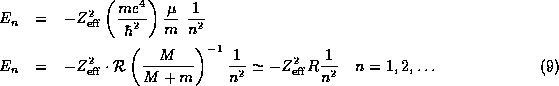
where the special combination of constants
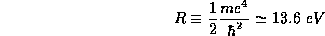
is given a special name, the Rydberg.
The spectrum of states may be plotted on an energy-level diagram as in Figure 1.2.
Note that the spacings decrease as infinitely many states
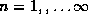 , accumulate below E = 0.
, accumulate below E = 0.