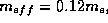 where
where
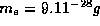 is the mass of an electron in free space.
is the mass of an electron in free space.
To make color displays with semiconductor devices, workers try to increase the energy of electrons several volts by confining the electrons in very small crystals.
The behavior of an electron moving through a crystal is much like that
of an electron moving through free space, except that because of
the interaction between the electron and the crystal the electron acts
as though it had a different mass, an ``effective mass''. In
Germanium, for instance, this mass is 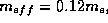 where
where
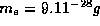 is the mass of an electron in free space.
is the mass of an electron in free space.
Check the feasibility of this approach in Germanium by using the
uncertainty principle to estimate the kinetic energy of an
electron of mass  confined to a cube of side L. How does the
energy of the electron vary as L decreases? What size L is needed
to increase the energy of the electron by 1 eV? Is this reasonable
or is it smaller than the average spacing between atoms in Germanium?
(The density of Germanium is 5.32
confined to a cube of side L. How does the
energy of the electron vary as L decreases? What size L is needed
to increase the energy of the electron by 1 eV? Is this reasonable
or is it smaller than the average spacing between atoms in Germanium?
(The density of Germanium is 5.32 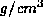 and its atomic weight is
72.6
and its atomic weight is
72.6 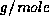 .)
.)