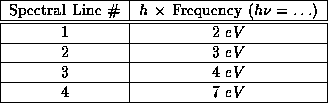
Table 2: Observed Spectral Lines for element X
Observations of the spectrum of element X show sharp emission lines at the frequencies in Table 2. ( This need not be a complete list of the lines in the spectrum.)
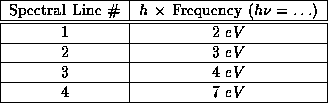
Table 2: Observed Spectral Lines for element X
On figure 1, using the least number of energy
states possible, sketch a set of energy levels which
explain the spectral lines listed in the table, next to each line
give the value of its energy (you may place the arbitrary
level of zero energy anywhere you wish), and then
answer the following question. From your energy
diagram, what are the frequencies of other spectral lines (if any)
that you might expect to see in the spectrum?
Hint: Note that there are several valid diagrams; you are only
required to give one example which produces the observed set of lines.

Figure 1: Inferred Energy States for element X