


Next: Comments
Up: No Title
Previous: Consequence of the
Note that Equation (3) predicts a shift in the
wavelength and frequency of the re-radiated light, an effect
unexpected in classical physics. Note also the the magnitude of the
shift in wavelength  is
predicted to be independent of the frequency of the incoming
radiation. It has the same value whether visible light, X-rays or
is
predicted to be independent of the frequency of the incoming
radiation. It has the same value whether visible light, X-rays or
 -rays are used. When using visible light
-rays are used. When using visible light
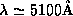 the shift is hardly noticeable, but as
one moves to the higher frequency and shorter wavelengths of X- and
the shift is hardly noticeable, but as
one moves to the higher frequency and shorter wavelengths of X- and
 rays, the shift becomes quite significant.
rays, the shift becomes quite significant.
These predictions are borne out the in experiments. Typically,
one sends 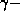 rays into a solid target. Because the
rays into a solid target. Because the
 rays have energies in excess of 1 MeV, the binding of
the electrons to the solid is relatively insignificant (at most
hundreds of keV for the most tightly bound electrons in the heaviest
nuclei) and we can ignore the electrons' motion within the solid.
Typical experimental results might appear as in Figure 3.
rays have energies in excess of 1 MeV, the binding of
the electrons to the solid is relatively insignificant (at most
hundreds of keV for the most tightly bound electrons in the heaviest
nuclei) and we can ignore the electrons' motion within the solid.
Typical experimental results might appear as in Figure 3.

Experimentally, one finds a peak centered at the incoming wavelength
(``in'' in the figure) and an additional peak shifted in exact accord
with (3) (``scatt'' in the figure). The peak at the
incoming wavelength is easy to understand from our result
(3). The photons in the experiment scatter off of
not only the electrons but also the nuclei in the solid. In addition
it is possible that when the photons hit a particularly tightly bound
electron, the recoil is taken up by the atom a whole and not by the
electron alone. Finally, events are also possible where the
entire crystal recoils. In these cases, one must use the mass of the
recoiling object in the formula for the Compton wavelength,  . As the mass of the nuclei and atoms in the target
are generally at least 40,000 times more massive than the electron,
the shift from these alternate scattering events is puny in comparison
to the shift from the electrons and does not show up in the
experimental results.
. As the mass of the nuclei and atoms in the target
are generally at least 40,000 times more massive than the electron,
the shift from these alternate scattering events is puny in comparison
to the shift from the electrons and does not show up in the
experimental results.



Next: Comments
Up: No Title
Previous: Consequence of the
Prof. Tomas Alberto Arias
Wed Oct 11 20:18:35 EDT 1995
 is
predicted to be independent of the frequency of the incoming
radiation. It has the same value whether visible light, X-rays or
is
predicted to be independent of the frequency of the incoming
radiation. It has the same value whether visible light, X-rays or
 -rays are used. When using visible light
-rays are used. When using visible light
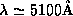 the shift is hardly noticeable, but as
one moves to the higher frequency and shorter wavelengths of X- and
the shift is hardly noticeable, but as
one moves to the higher frequency and shorter wavelengths of X- and
 rays, the shift becomes quite significant.
rays, the shift becomes quite significant.
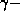 rays into a solid target. Because the
rays into a solid target. Because the
 rays have energies in excess of 1 MeV, the binding of
the electrons to the solid is relatively insignificant (at most
hundreds of keV for the most tightly bound electrons in the heaviest
nuclei) and we can ignore the electrons' motion within the solid.
Typical experimental results might appear as in Figure
rays have energies in excess of 1 MeV, the binding of
the electrons to the solid is relatively insignificant (at most
hundreds of keV for the most tightly bound electrons in the heaviest
nuclei) and we can ignore the electrons' motion within the solid.
Typical experimental results might appear as in Figure 
 . As the mass of the nuclei and atoms in the target
are generally at least 40,000 times more massive than the electron,
the shift from these alternate scattering events is puny in comparison
to the shift from the electrons and does not show up in the
experimental results.
. As the mass of the nuclei and atoms in the target
are generally at least 40,000 times more massive than the electron,
the shift from these alternate scattering events is puny in comparison
to the shift from the electrons and does not show up in the
experimental results.