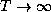 )? Why?
)? Why?
Consider a three-state system as indicated in the diagram in Figure 2. Here the first two states, 1 and 2, have the same energy. (This situation is referred to as a ``degeneracy''.)
a) What do you expect the average energy of this system to be at
absolute zero temperature (T=0)? At extremely high temperatures
(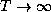 )? Why?
)? Why?
b) To confirm your expectations, apply the Boltzmann distribution to
write the probability of each of the three states as 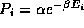 , where
, where  is the proportionality constant and
is the proportionality constant and
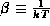 , where k is Boltzmann's constant. Use
the condition that these probabilities must sum to unity (the
normalization of the probability distribution) to determine
, where k is Boltzmann's constant. Use
the condition that these probabilities must sum to unity (the
normalization of the probability distribution) to determine
 . Finally, compute the average energy of this system at
temperature T and verify your predictions in a) for the
behavior of this system as
. Finally, compute the average energy of this system at
temperature T and verify your predictions in a) for the
behavior of this system as 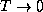 and
and 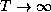 .
.
c) Confirm that the shortcut of summing the partition
function, 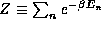 , and then taking
, and then taking

gives the correct result.